The Historical Evolution of the Periodic Table of Chemical Elements
- Er. Akhil A R

- Mar 28, 2025
- 23 min read
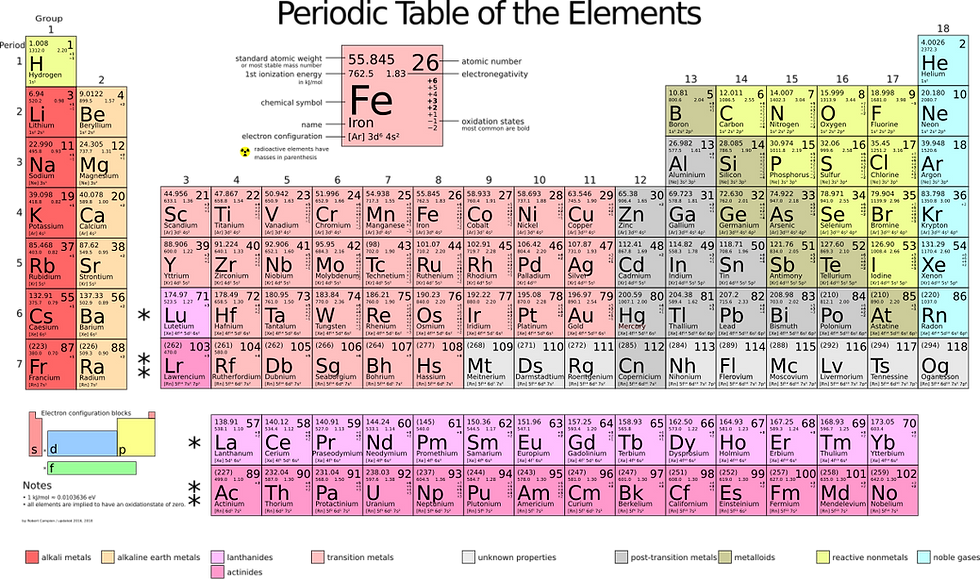
The Significance of the Periodic Table in Chemistry
The periodic table of chemical elements stands as a monumental achievement in the history of science, serving as a cornerstone of modern chemistry 1. It is more than just a tabular arrangement; it represents a profound understanding of the fundamental building blocks of matter and the intricate relationships between them 2. This systematic organization of the known elements has not only provided a framework for understanding their diverse properties but has also served as a powerful tool for predicting the existence and characteristics of undiscovered elements and guiding research across various scientific disciplines 3. The journey to this elegant and informative system was a long and winding one, marked by the contributions of numerous scientists who, through their observations, experiments, and insights, gradually unveiled the underlying order of the chemical world. This report will trace the complete history of the periodic table, from its earliest conceptualizations to its modern form, detailing the key milestones, the scientists who shaped its development, and the pivotal discoveries that led to our current understanding of the elements and their organization.
Early Steps Towards Classification: Laying the Groundwork
The quest to understand and organize the fundamental substances of the universe, the chemical elements, began long before the formal development of the periodic table. Early chemists recognized the need for a system to classify the growing number of known elements, and several pioneering attempts laid the groundwork for later, more comprehensive systems.
A. Lavoisier's Initial Classification
Towards the end of the 18th century, as chemistry began to emerge as a more quantitative science, Antoine Lavoisier made an initial attempt to bring order to the known elements 5. Around the year 1800, approximately 30 elements had been identified, and Lavoisier proposed a classification based on fundamental observable properties 4. His system primarily divided these elements into two broad categories: metals and nonmetals 5. This binary classification, while rudimentary by today's standards, represented an important first step in recognizing that elements could be grouped based on shared characteristics 5. The distinction between metals, typically exhibiting properties like luster, malleability, and conductivity, and nonmetals, often characterized by their brittleness and poor conductivity, provided a basic framework for organizing the increasing chemical knowledge 5. However, this early system had its limitations. It struggled to accommodate elements that exhibited properties of both metals and nonmetals, highlighting the need for a more nuanced and comprehensive approach to classification as more elements with diverse characteristics were discovered 5.
B. Johann Wolfgang Döbereiner's Triads (1817-1829)
In the early 19th century, Johann Wolfgang Döbereiner, a German chemist, embarked on a more refined attempt to classify elements by observing patterns within smaller groups 2. Between 1817 and 1829, Döbereiner noticed that certain elements with similar chemical and physical properties tended to occur in groups of three, which he termed "triads" 2. One of the most notable triads he identified consisted of the alkali metals: lithium (Li), sodium (Na), and potassium (K) 5. He observed that these three elements shared similar chemical behaviors, such as reacting vigorously with water to form strong alkalis 5. Furthermore, Döbereiner noted a mathematical relationship in their atomic weights: the atomic weight of sodium (approximately 23) was roughly the arithmetic mean of the atomic weights of lithium (approximately 7) and potassium (approximately 39) 2. Another significant triad identified by Döbereiner comprised the alkaline earth metals: calcium (Ca), strontium (Sr), and barium (Ba) 2. Similar to the alkali metals, these elements exhibited analogous chemical properties, and the atomic weight of strontium (approximately 88) was close to the average of the atomic weights of calcium (approximately 40) and barium (approximately 137) 2. Döbereiner also recognized a triad among the halogens: chlorine (Cl), bromine (Br), and iodine (I) 2. These elements shared similar reactivity and formed salts with metals, and again, the atomic weight of bromine (approximately 80) was near the average of chlorine (approximately 35.5) and iodine (approximately 127) 5. While Döbereiner identified several such triads, including sulfur, selenium, and tellurium, his model had inherent limitations 6. Not all of the approximately 53 elements known at the time could be easily grouped into triads 5. Moreover, as new elements were discovered, many did not fit into the triad pattern, rendering the model increasingly inadequate 2. Additionally, with more precise measurements of atomic masses, the arithmetic mean rule was not strictly applicable to all potential triads, particularly for elements with very low or very high atomic masses, such as fluorine, chlorine, and bromine 6. Despite these shortcomings, Döbereiner's work was a crucial step forward. It was among the earliest attempts to establish a logical order among elements based on their properties and atomic weights, suggesting an underlying relatedness that would be further explored by subsequent scientists 5.
C. John Newlands' Law of Octaves (1864) 2
Building upon the foundation laid by Döbereiner and others, English chemist John Newlands made another significant attempt at classification in 1864 2. By this time, the number of known elements had grown to around 62 9. Newlands arranged these elements in ascending order of their atomic weights, a concept that was gaining prominence as a fundamental property of elements 2. In his arrangement, Newlands observed a recurring pattern: every eighth element exhibited similar chemical and physical properties 2. Drawing an analogy to the musical scale, where every eighth note repeats the first, Newlands termed this observation the "Law of Octaves" 2. For instance, he noted that lithium (the first element in his arrangement) shared similarities with sodium (the eighth element), and sodium with potassium (the fifteenth element), and so on 2. Similarly, fluorine was observed to have properties akin to chlorine, the eighth element after it 2. Newlands presented his Law of Octaves in 1865, but it was met with considerable skepticism and even ridicule by some members of the scientific community 12. One of the major limitations of Newlands' law was that it did not hold true for elements beyond calcium (the 20th element in his ordering) 9. Elements with higher atomic weights did not consistently fall into groups with similar properties every eight elements 9. Furthermore, in order to fit all the known elements into his octave pattern, Newlands was forced to place some elements with distinctly different properties into the same group 9. For example, he placed iron in the same group as oxygen and sulfur, despite their vastly different chemical behaviors 5. He also did not account for the possibility of undiscovered elements, assuming that all the elements that existed were already known 2. Despite the criticisms and limitations, Newlands' work was a significant step in the development of the periodic table 9. He was one of the first to explicitly recognize the periodic recurrence of elemental properties based on their atomic weights, a fundamental concept that would be central to Mendeleev's later success 11. His attempt, though flawed, highlighted the potential for a systematic arrangement of elements based on this fundamental property.
Mendeleev's Groundbreaking Contribution: The Periodic Law Emerges
The true breakthrough in the classification of elements came in 1869 with the work of the Russian chemist Dmitri Ivanovich Mendeleev 1. Driven by the need for a better way to organize the elements for his textbook, "Principles of Chemistry," Mendeleev embarked on a systematic study of the relationships between the elements 2.
A. Dmitri Mendeleev's Approach (1869)
Mendeleev's key insight was that when all the known chemical elements (approximately 70 at the time) were arranged in order of increasing atomic weight, they exhibited a recurring pattern, or periodicity, of properties 2. He presented his findings to the Russian Chemical Society in March 1869, stating that "elements arranged according to the value of their atomic weights present a clear periodicity of properties" 15. Unlike his predecessors, Mendeleev was not afraid to break the strict order of increasing atomic weights when necessary to place elements with similar chemical properties into the same vertical columns, or groups, in his table 3. For instance, he placed iodine after tellurium, even though iodine has a slightly lower atomic weight, because iodine's properties were more similar to those of fluorine, chlorine, and bromine in the halogen group 19. Around the same time, Lothar Meyer, a German chemist, also developed a periodic table very similar to Mendeleev's 3. However, because Mendeleev's work was published slightly earlier and was more comprehensive in its predictions, he is generally credited as the primary architect of the periodic table 3. Mendeleev's initial table arranged the elements in rows based on increasing atomic weight, with elements sharing similar valency and chemical behavior falling into the same columns 16. This arrangement allowed him to recognize not only existing relationships but also to identify gaps where elements with specific predicted properties should exist 3.
B. Formulation of the Periodic Law
Mendeleev's observation led to the formulation of the periodic law, which, in its initial form, stated that the properties of the elements are a periodic function of their atomic weights 2. This law provided a fundamental framework for understanding the organization of matter, suggesting that the chemical behavior of elements was not random but was intrinsically linked to their atomic weight and followed a predictable pattern 1. The periodic law was a significant departure from earlier attempts at classification, as it not only grouped known elements but also implied the existence of a deeper, underlying order in the natural world 1.
C. Mendeleev's Predictions of Undiscovered Elements
One of the most remarkable aspects of Mendeleev's periodic table was the deliberate inclusion of gaps 2. Rather than viewing these gaps as flaws, Mendeleev boldly predicted that they represented elements that were yet to be discovered 3. Furthermore, based on the position of these gaps within his table and the periodic trends he observed, Mendeleev went on to predict the physical and chemical properties of these "missing" elements, including their atomic weights, densities, melting points, and how they would react with other substances 3. He even gave these predicted elements temporary names using the Sanskrit prefixes "eka-", "dvi-", and "tri-" to indicate their position relative to known elements in the same group 16. For example, he predicted the existence of "eka-boron" (which would fall below boron), "eka-aluminium" (below aluminium), and "eka-silicon" (below silicon) 16. Eka-boron was later discovered and named scandium (Sc), and its properties closely matched Mendeleev's predictions 16. Similarly, eka-aluminium was discovered as gallium (Ga) in 1875, and its properties, such as an atomic mass of approximately 69.3 and a low melting point, were remarkably close to Mendeleev's predictions for an element with an atomic mass of 68 3. Eka-silicon was discovered as germanium (Ge) in 1886, and again, its properties aligned well with Mendeleev's predictions for an element with an atomic mass of 72 3. Mendeleev also predicted other elements, such as eka-manganese (which was later identified as technetium, though discovered much later through artificial means), dvi-tellurium (polonium), and tri-manganese (rhenium) 22. These bold predictions and their subsequent confirmation were instrumental in the widespread acceptance of Mendeleev's periodic table and established it as a powerful and predictive tool in chemistry 3.
Initial Reception and Challenges to Mendeleev's Table: Overcoming Skepticism
Despite the profound insights and predictive power of Mendeleev's periodic table, it was not immediately embraced by the entire scientific community 4. New scientific ideas often face initial skepticism, and Mendeleev's work was no exception.
Skepticism and Initial Lack of Widespread Acceptance
Initially, some scientists were hesitant to accept Mendeleev's periodic table 4. The very idea of predicting the existence and properties of undiscovered elements was considered audacious by some 16. Furthermore, the fact that Mendeleev had to occasionally deviate from the strict ordering of elements by atomic weight to maintain groups with similar properties raised questions about the fundamental basis of his system 3. The lack of a clear theoretical explanation for why the elements exhibited this periodicity also contributed to the initial skepticism 18. While Lothar Meyer had independently developed a similar table, the fact that Mendeleev's work was published first and included these bold predictions ultimately led to him receiving the majority of the credit 3.
Eventual Validation Through the Discovery of Predicted Elements
The turning point for the acceptance of Mendeleev's periodic table came with the discovery of the elements he had predicted 3. The discovery of gallium in 1875, germanium in 1886, and scandium in 1879, and the remarkable agreement between their observed properties and Mendeleev's predictions, provided compelling evidence for the validity and predictive power of his periodic law 3. For instance, the properties of gallium, discovered by French chemist Paul-Émile Lecoq de Bioburden, matched almost exactly with Mendeleev's predictions for eka- aluminium, including its atomic mass, density, melting point, and the formula of its oxide 20. This striking confirmation of his predictions silenced many of the initial critics and led to the widespread recognition of the periodic table as a fundamental organizing principle in chemistry.
Anomalies and Inconsistencies in Mendeleev's Table
Despite its success, Mendeleev's periodic table was not without its challenges and inconsistencies 19. One notable issue was the reversed order of some elements based on their atomic weights 19. For example, as mentioned earlier, Mendeleev placed iodine after tellurium, and nickel after cobalt, even though tellurium and cobalt had slightly higher atomic weights than iodine and nickel, respectively 19. He did this to ensure that these elements were grouped with others exhibiting similar chemical properties 19. Another challenge was the placement of hydrogen, which exhibited properties similar to both the alkali metals (Group 1) and the halogens (Group 17), making its position in the table somewhat ambiguous 20. Furthermore, the existence of isotopes, atoms of the same element with different atomic masses (discovered later in the early 20th century), posed a problem for a classification system based solely on atomic weight 20. Isotopes of an element have the same chemical properties but different masses, raising the question of where they should be placed in the table 20. Additionally, Mendeleev initially resisted the inclusion of the noble gases upon their discovery 26. These anomalies suggested that while Mendeleev's framework was remarkably accurate, atomic weight alone might not be the ultimate organizing principle for the periodic table 19.
The Discovery of Noble Gases: Expanding the Periodic System
The late 19th century witnessed the discovery of an entirely new group of elements: the noble gases 3. These elements, characterized by their extreme lack of chemical reactivity, significantly expanded the periodic system and provided further insights into the nature of chemical periodicity.
Historical Context of Their Discovery (1785-1904)
The story of the noble gases began in 1785 when Henry Cavendish observed a small, unreactive residue in the air after removing nitrogen and oxygen 31. However, this observation was largely overlooked for about a century 31. In 1868, during a solar eclipse, a new spectral line was observed in the Sun's chromosphere and attributed to a previously unknown element named helium (from the Greek word for the Sun, "helios") 31. It was not until 1895 that William Ramsay successfully isolated helium on Earth by heating the mineral cleveite 32. The discovery of argon in 1894 by Lord Rayleigh and William Ramsay marked the recognition of an entirely new class of gases 31. Rayleigh had noticed that nitrogen extracted from the air had a slightly higher density than nitrogen produced from chemical reactions, leading him to suspect the presence of another, denser gas 32. Collaboration with Ramsay led to the isolation of this new element, which they named argon (from the Greek word "argos," meaning "idle" or "lazy," reflecting its inert nature) 32. Following the discovery of argon, Ramsay continued his search for similar gases by using the method of fractional distillation of liquid air 32. In 1898, he successfully isolated three more noble gases: krypton (from the Greek word "kryptos," meaning "hidden"), neon (from "neos," meaning "new"), and xenon (from "xenos," meaning "stranger") 30. The final naturally occurring noble gas, radon, was identified in 1900 by Friedrich Ernst Dorn and was initially called radium emanation 30. Its properties were later found to be similar to those of the other noble gases, and it was established as a member of the group in 1904.
Their Unique Properties and Why They Were Initially Missed
The noble gases possess a unique set of properties that led to their delayed discovery 31. Their most defining characteristic is their extreme chemical inertness; they tend not to react with other elements to form chemical compounds under normal conditions 32. This lack of reactivity stems from their electronic configuration: they have a full outer shell of valence electrons, making them exceptionally stable and disinclined to gain, lose, or share electrons 32. Because early chemistry heavily focused on the reactions between elements to form compounds, these non-reactive gases remained largely undetected 31. They were initially referred to as "inert gases" or "rare gases" due to their perceived scarcity and lack of reactivity 32. However, it is now known that some noble gases, like argon, are quite abundant in the Earth's atmosphere 32. The term "noble gases" is now preferred, drawing an analogy to "noble metals" like gold and platinum, which also exhibit low reactivity 32.
The Impact of Noble Gas Discovery on the Periodic Table and Their Placement (Group 0/18)
The discovery of the noble gases had a significant impact on the periodic table 3. Their existence necessitated an expansion of Mendeleev's original table to accommodate this entirely new group of elements 3. Initially, they were placed in a new column designated as Group 0, reflecting their perceived zero valence or lack of reactivity 32. Mendeleev himself eventually included the noble gases in his table in this Group 0 after accepting the evidence for helium and argon 17. In the modern periodic table, the noble gases are located in Group 18, the far right column 32. This placement reflects their full outer electron shells and their position at the end of each period, after the most reactive nonmetals (halogens) 33. The noble gases fit remarkably well into the periodic trends based on their atomic weights and electronic configurations 32. Their discovery further validated the underlying principles of the periodic table, demonstrating its ability to accommodate new discoveries and highlighting the importance of electronic structure in determining chemical properties 32. While initially thought to be completely inert, it was later discovered that heavier noble gases like xenon can indeed form compounds with highly electronegative elements like fluorine, further refining our understanding of their chemistry 33.
The Modern Periodic Table: The Role of Atomic Number
The early 20th century brought a crucial refinement to the periodic table with the work of the English physicist Henry Moseley 19. His experiments with X-ray spectroscopy provided a more fundamental basis for the ordering of elements.
Henry Moseley's Experiments with X-ray Spectroscopy (1913-1914)
Between 1913 and 1914, Henry Moseley conducted a series of groundbreaking experiments using X-ray spectroscopy 19. He systematically bombarded various elements with electrons in an X-ray tube and measured the wavelengths of the emitted X-rays using crystal diffraction 29. Moseley discovered a direct mathematical relationship between the frequency (or wavelength) of the emitted X-rays and the atomic number of the element 19. Specifically, he found that the square root of the frequency of a particular X-ray line was approximately proportional to the atomic number (Z) of the element 42. This relationship became known as Moseley's Law 19. Prior to Moseley's work, atomic number was merely considered the element's position in the periodic table, loosely related to atomic weight but not directly linked to any measurable physical quantity 19. Moseley's experiments provided the first direct experimental determination of atomic number, establishing it as a fundamental property of an element related to the charge of its nucleus 19.
Establishing Atomic Number as the Fundamental Organizing Principle
Moseley's findings conclusively demonstrated that the atomic number, representing the number of protons in the nucleus of an atom, was the true basis for the periodic law, rather than atomic weight 19. This confirmed an earlier hypothesis by Antonius van den Broek, who had suggested that the atomic number corresponded to the positive charge of the nucleus 39. Moseley's work provided experimental evidence to support this idea and offered a precise method for determining the atomic number of each element 19. This shift from atomic weight to atomic number as the organizing principle provided a more accurate and theoretically sound foundation for the periodic table, aligning the chemical behavior of elements with their fundamental atomic structure 19.
Resolution of Anomalies in Mendeleev's Table
Moseley's discovery elegantly resolved the anomalies that existed in Mendeleev's periodic table, where some elements were placed out of order based on atomic weight to fit their chemical properties 19. For instance, tellurium (atomic number 52) has a slightly higher atomic mass than iodine (atomic number 53), but Moseley's work showed that iodine should indeed follow tellurium in the periodic table based on its higher atomic number, consistent with their chemical properties 19. Similarly, cobalt (atomic number 27) has a slightly higher atomic mass than nickel (atomic number 28), but their placement in the periodic table with cobalt preceding nickel aligns with their atomic numbers and chemical behavior 19. These corrections based on atomic number provided strong support for the modern arrangement of the periodic table 19.
Moseley's Prediction of Missing Elements
Moseley's experiments also revealed gaps in the atomic number sequence, indicating the existence of undiscovered elements 39. He predicted the atomic numbers of these missing elements to be 43, 61, 72, and 75, which correspond to the elements technetium (Tc), promethium (Pm), hafnium (Hf), and rhenium (Re), respectively 39. These predictions further underscored the power of the periodic table organized by atomic number as a tool for guiding scientific discovery 39. Moseley also correctly determined that there were exactly 15 lanthanide elements, resolving a long-standing question among chemists 39. Tragically, Moseley's promising scientific career was cut short when he died in World War I at the young age of 27 19. His work, however, laid the foundation for the modern periodic table and our understanding of the fundamental nature of chemical elements.
The Expansion Beyond Uranium: Transuranium Elements
The periodic table continued to expand beyond the naturally occurring elements with the discovery of the transuranium elements, those with atomic numbers greater than uranium (Z=92) 22.
Discovery of Neptunium, Plutonium, and Subsequent Transuranium Elements
The discovery of transuranium elements began in the mid-20th century, primarily through nuclear reactions 22. Neptunium (Np, Z=93) was the first transuranium element to be synthesized in 1940 by bombarding uranium with neutrons 22. Shortly thereafter, in the same year, plutonium (Pu, Z=94) was also synthesized 22. These discoveries demonstrated that the periodic table was not limited to the elements found naturally on Earth and that new elements could be created through human intervention 22. This opened up a new frontier in chemistry and physics, leading to the synthesis of numerous other transuranium elements, extending the periodic table to its current form.
Methods of Synthesis and Their Placement in the Periodic Table (Actinide series)
Transuranium elements are typically synthesized by bombarding heavy atomic nuclei with other particles, such as neutrons or other nuclei, in nuclear reactors or particle accelerators 22. These nuclear reactions can lead to the formation of new elements with higher atomic numbers. These synthetic elements are placed in the periodic table following uranium. The elements from atomic number 90 (thorium) to 103 (lawrencium) form the actinide series, which is typically placed below the main body of the periodic table, similar to the lanthanide series (elements 57 to 71) 22. This placement reflects their electronic configurations, particularly the filling of the f-orbitals, and their shared chemical properties 22. The discovery and synthesis of transuranium elements have significantly expanded our understanding of nuclear chemistry and the limits of the periodic table.
A Historical Timeline of Element Discovery
The discovery of chemical elements has been a gradual process spanning centuries, with the pace of discovery accelerating alongside advancements in scientific understanding and technology 2.
Major Periods and Advancements that Facilitated Element Discovery
The history of element discovery can be broadly divided into several periods, each characterized by specific scientific advancements that enabled the identification of new elements:
Antiquity to the 17th Century: This period saw the discovery of elements known since ancient times, primarily metals that occurred in their native state or were easily extracted from their ores. Examples include gold (Au), silver (Ag), copper (Cu), iron (Fe), lead (Pb), mercury (Hg), and tin (Sn) 4.
18th Century: The 18th century marked a significant period of advancement in chemistry, with a greater understanding of gases and combustion. This led to the discovery of several gaseous elements, including hydrogen (H), oxygen (O), and nitrogen (N), as well as other elements like chlorine (Cl) and phosphorus (P) 4. The recognition of elements as fundamental substances distinct from compounds also gained traction during this time 5.
Early to Mid-19th Century: The development of electrochemistry in the early 19th century, pioneered by scientists like Humphry Davy, provided a powerful new tool for isolating elements. This led to the discovery of highly reactive alkali metals such as sodium (Na) and potassium (K), as well as alkaline earth metals like calcium (Ca) and magnesium (Mg) 2. Increased accuracy in determining atomic weights also played a crucial role in the classification efforts of Döbereiner and others, indirectly facilitating the recognition of new elements like bromine (Br) and iodine (I) 2.
Late 19th Century: The advent of spectroscopy in the mid-19th century, with the work of Robert Bunsen and Gustav Kirchhoff, revolutionized element discovery. Each element was found to emit a unique pattern of light when heated, allowing for their identification even in trace amounts. This technique led to the discovery of elements like cesium (Cs), rubidium (Rb), thallium (Tl), indium (In), gallium (Ga), and germanium (Ge) 2. The careful analysis of gases by Lord Rayleigh and William Ramsay in the late 19th century resulted in the discovery of the entire group of noble gases: argon (Ar), helium (He), neon (Ne), krypton (Kr), and xenon (Xe) 2.
Early to Mid-20th Century: The early 20th century saw the discovery of many radioactive elements, some of which filled the gaps predicted by Mendeleev and Moseley. Techniques for isolating and identifying these unstable elements advanced significantly during this period. The development of nuclear physics in the mid-20th century led to the synthesis of the first transuranium elements, starting with neptunium and plutonium 3.
Late 20th and Early 21st Century: The latter part of the 20th and the beginning of the 21st century have been marked by the synthesis of superheavy elements with very high atomic numbers, often requiring sophisticated particle accelerators and detection methods.
Table: Timeline of Element Discovery
The following table summarizes the major periods and advancements in the history of element discovery:
Period | Key Scientific Advancements | Examples of Elements Discovered |
Antiquity to 17th Century | Early metallurgy, basic chemical observations | Gold (Au), Silver (Ag), Copper (Cu), Iron (Fe), Lead (Pb), Mercury (Hg) |
18th Century | Understanding of gases, combustion theory | Hydrogen (H), Oxygen (O), Nitrogen (N), Chlorine (Cl), Phosphorus (P) |
Early to Mid-19th Century | Development of electrochemistry, more accurate atomic weights | Sodium (Na), Potassium (K), Calcium (Ca), Magnesium (Mg), Bromine (Br), Iodine (I) |
Late 19th Century | Development of spectroscopy, careful gas analysis | Cesium (Cs), Rubidium (Rb), Thallium (Tl), Indium (In), Gallium (Ga), Germanium (Ge), Argon (Ar), Helium (He), Neon (Ne), Krypton (Kr), Xenon (Xe) |
Early to Mid-20th Century | Understanding of radioactivity, development of nuclear physics | Technetium (Tc), Promethium (Pm), Hafnium (Hf), Rhenium (Re), Neptunium (Np), Plutonium (Pu) |
Late 20th and Early 21st Century | Advanced particle accelerators, sophisticated detection methods | Bohrium (Bh), Darmstadtium (Ds), Roentgenium (Rg), Copernicium (Cn), Nihonium (Nh), Flerovium (Fl), Moscovium (Mc), Livermorium (Lv), Tennessine (Ts), Oganesson (Og) |
The Significance and Predictive Power of the Periodic Table
The periodic table is far more than just a historical artifact; it is a fundamental and indispensable tool in modern chemistry, providing a framework for understanding and predicting the properties and behavior of elements 4.
Understanding Periodic Trends in Element Properties 4
The arrangement of elements in the periodic table reveals systematic trends in their physical and chemical properties based on their position within the table 4. Moving from left to right across a period (row), properties like atomic size generally decrease due to increasing nuclear charge and effective attraction for electrons. Ionization energy, the energy required to remove an electron, generally increases across a period as the electrons are held more tightly. Electronegativity, a measure of an atom's ability to attract electrons in a chemical bond, also generally increases across a period. Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period, with nonmetals appearing on the right side. Conversely, moving down a group (column), atomic size generally increases due to the addition of electron shells. Ionization energy and electronegativity generally decrease down a group as the outermost electrons are further from the nucleus and shielded by inner electrons. Metallic character generally increases down a group, with metals appearing at the bottom of many groups. Reactivity also often follows predictable trends; for example, alkali metals (Group 1) become more reactive as you move down the group, while halogens (Group 17) become less reactive. These periodic trends are a direct consequence of the electronic structure of the atoms and the way electrons are arranged in different energy levels and orbitals 4.
The Periodic Table as a Predictive Tool for Chemical Behavior and Compound Formation 4
The periodic table's organization makes it a powerful predictive tool for understanding how elements will behave chemically and what types of compounds they are likely to form 4. Elements within the same group have similar numbers of valence electrons (electrons in the outermost shell), which largely determine their chemical reactivity 32. For example, elements in Group 1 (alkali metals) all have one valence electron and tend to lose this electron to form +1 ions, making them highly reactive with nonmetals like halogens (Group 17), which have seven valence electrons and tend to gain one electron to form -1 ions. This understanding allows us to predict that sodium (Na) from Group 1 will react with chlorine (Cl) from Group 17 to form sodium chloride (NaCl) with a 1:1 stoichiometry. Similarly, elements in Group 2 (alkaline earth metals) have two valence electrons and typically form +2 ions. The periodic table also helps predict the oxidation states that elements are likely to exhibit in their compounds. The position of an element can provide clues about the types of bonds it will form (ionic or covalent) and the formulas of the resulting compounds. For instance, knowing that oxygen is in Group 16 and typically forms -2 ions, and that aluminum is in Group 13 and typically forms +3 ions, we can predict that they will combine to form aluminum oxide with the formula Al₂O₃ to balance the charges. This predictive capability makes the periodic table an invaluable resource for chemists in designing experiments, synthesizing new materials, and understanding chemical reactions 4.
The Enduring Legacy of the Periodic Table
The history of the periodic table is a testament to the power of scientific inquiry and the gradual unfolding of nature's order. From the early attempts of Lavoisier, Döbereiner, and Newlands to the groundbreaking work of Mendeleev, and the crucial refinements by Moseley, each step contributed to our understanding of the fundamental building blocks of the universe 2. Mendeleev's genius in recognizing the periodic law and boldly predicting undiscovered elements laid the foundation for the modern table 2. The subsequent discovery of noble gases expanded the system, and Moseley's work establishing atomic number as the organizing principle provided a more fundamental and accurate arrangement 3. The expansion beyond uranium with the synthesis of transuranium elements further demonstrated the dynamic nature of the periodic table and our ever-growing knowledge of matter 22. Today, the periodic table remains a cornerstone of chemistry, providing not only a systematic organization of the elements but also a powerful tool for predicting their properties and guiding scientific research and education across the globe 4. Its enduring legacy lies in its ability to reveal the fundamental unity and order within the vast diversity of the chemical world.
References
Mendeleev's Legacy: The Periodic System - Science History Institute, accessed March 28, 2025, https://www.sciencehistory.org/stories/magazine/mendeleevs-legacy-the-periodic-system/
solarsystem.nasa.gov, accessed March 28, 2025, https://solarsystem.nasa.gov/genesismission/educate/scimodule/UnderElem/UnderElem_pdf/HistOverST.pdf
READ: Dmitri Mendeleev (article) - Khan Academy, accessed March 28, 2025, https://www.khanacademy.org/humanities/big-history-project/stars-and-elements/knowing-stars-elements/a/dmitri-mendeleev
Compare-Contrast-Connect: The History of Mendeleev's Table | manoa.hawaii.edu/ExploringOurFluidEarth, accessed March 28, 2025, https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/nature-and-organization-elements/compare-contrast-connect-history-mendeleevs-table
Early Attempts at the Classification of Elements: Dobereiner, Newlands - EMBIBE, accessed March 28, 2025, https://www.embibe.com/exams/early-attempts-at-the-classification-of-elements/
Döbereiner's triads - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/D%C3%B6bereiner%27s_triads
Early History of the Periodic Table - Methods, Properties, Laws, Challenges and Development | CK-12 Foundation, accessed March 28, 2025, https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/6.1/primary/lesson/early-history-of-the-periodic-table-chem/
2.11: Early History of the Periodic Table - Chemistry LibreTexts, accessed March 28, 2025, https://chem.libretexts.org/Courses/University_of_Pittsburgh_at_Bradford/CHEM_0106%3A_Chemistry_of_the_Environment/02%3A_Chemical_Elements/2.11%3A_Early_History_of_the_Periodic_Table
Newland's Law of Octaves and Dobereiner's Triads - BYJU'S, accessed March 28, 2025, https://byjus.com/chemistry/newlands-law-octaves/
byjus.com, accessed March 28, 2025, https://byjus.com/chemistry/newlands-law-octaves/#:~:text=On%20the%20basis%20of%20this,order%20of%20their%20atomic%20masses.
Law of octaves | Definition & Facts - Britannica, accessed March 28, 2025, https://www.britannica.com/science/law-of-octaves
Newlands Law of Octaves - Simply Science, accessed March 28, 2025, https://www.simply.science/images/content/chemistry/structure_of_matter/dev_of_periodic_table/conceptmap/Newlands_Law.html
Newland's Law of Octaves: Definition, Examples, and FAQs - GeeksforGeeks, accessed March 28, 2025, https://www.geeksforgeeks.org/newlands-law-of-octaves/
www.britannica.com, accessed March 28, 2025, https://www.britannica.com/biography/Dmitri-Mendeleev#:~:text=Petersburg%2C%20Russia)%20was%20a%20Russian,properties%20within%20groups%20of%20elements.
Dmitri Mendeleev | Biography, Periodic Table, & Facts - Britannica, accessed March 28, 2025, https://www.britannica.com/biography/Dmitri-Mendeleev
Dmitri Mendeleev - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/Dmitri_Mendeleev
Mendeleev's Periodic Table - Origins: Current Events in Historical Perspective, accessed March 28, 2025, https://origins.osu.edu/milestones/mendeleev-periodic-table-UN-chemistry-radioactivity-noble-gases
Mendeleev's Periodic Table presented in public | OUPblog, accessed March 28, 2025, https://blog.oup.com/2012/03/mendeleev-periodic-table/
Moseley's Periodic Table - Corrosion-doctors.org, accessed March 28, 2025, https://corrosion-doctors.org/Periodic/Periodic-Moseley.htm
Mendeleev's Periodic Table: Achievements and Limitations - Study'n'Learn, accessed March 28, 2025, https://studynlearn.com/blog/mendeleevs-periodic-table/
www.khanacademy.org, accessed March 28, 2025, https://www.khanacademy.org/humanities/big-history-project/stars-and-elements/knowing-stars-elements/a/dmitri-mendeleev#:~:text=Gallium%2C%20germanium%2C%20and%20scandium%20were,basic%20characteristics%20Mendeleev%20had%20recorded.
Mendeleev's predicted elements - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/Mendeleev%27s_predicted_elements
Mendeleev's predicted elements - chemeurope.com, accessed March 28, 2025, https://www.chemeurope.com/en/encyclopedia/Mendeleev%27s_predicted_elements.html
What made Mendeleev's periodic table more widely accepted than others? - Socratic, accessed March 28, 2025, https://socratic.org/questions/what-made-mendeleev-s-periodic-table-more-widely-accepted-than-others
manoa.hawaii.edu, accessed March 28, 2025, https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/nature-and-organization-elements/compare-contrast-connect-history-mendeleevs-table#:~:text=Although%20some%20scientists%20were%20skeptical,accepted%20by%20the%20scientific%20community.
Mendeleev — Early Periodic Table & Periodic Law - Expii, accessed March 28, 2025, https://www.expii.com/t/mendeleev-early-periodic-table-periodic-law-11049
acshist.scs.illinois.edu, accessed March 28, 2025, https://acshist.scs.illinois.edu/bulletin_open_access/v47-1/v47-1%20p15-28.pdf
Anomalies of Mendeleev's Periodic Table - GeeksforGeeks, accessed March 28, 2025, https://www.geeksforgeeks.org/anomalies-of-mendeleevs-periodic-table/
Moseley's Periodic Law - Simply Science, accessed March 28, 2025, https://www.simply.science/images/content/chemistry/structure_of_matter/dev_of_periodic_table/conceptmap/Moseleys_Periodic_law.html
www.britannica.com, accessed March 28, 2025, https://www.britannica.com/science/noble-gas#:~:text=Ramsay%20and%20his%20coworkers%20searched,noble%2Dgas%20group%20in%201904.
Noble Gases | Oxford Research Encyclopedia of Planetary Science, accessed March 28, 2025, https://oxfordre.com/planetaryscience/display/10.1093/acrefore/9780190647926.001.0001/acrefore-9780190647926-e-33?d=%2F10.1093%2Facrefore%2F9780190647926.001.0001%2Facrefore-9780190647926-e-33&p=emailAI42AXHRM8rS6
Noble gas - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/Noble_gas
Noble gas | Definition, Elements, Properties, Characteristics, & Facts | Britannica, accessed March 28, 2025, https://www.britannica.com/science/noble-gas
The Incredible Discovery of the LEAST Reactive Elements (The Noble Gases) - YouTube, accessed March 28, 2025, https://www.youtube.com/watch?v=loqudG71uBM
William Ramsay | Science History Institute, accessed March 28, 2025, https://www.sciencehistory.org/education/scientific-biographies/william-ramsay/
Noble Gases - Periodic Table - ChemTalk, accessed March 28, 2025, https://chemistrytalk.org/noble-gases-periodic-table/
chemistrytalk.org, accessed March 28, 2025, https://chemistrytalk.org/noble-gases-periodic-table/#:~:text=They%20are%20members%20of%20group,decay%20of%20radioactive%20potassium%2D40.
Group 18: Properties of Nobel Gases - Chemistry LibreTexts, accessed March 28, 2025, https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_18%3A_The_Noble_Gases/1Group_18%3A_Properties_of_Nobel_Gases
Henry Moseley - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/Henry_Moseley
Henry Moseley and the Periodic Table of the Elements | NIST, accessed March 28, 2025, https://www.nist.gov/news-events/news/2024/02/henry-moseley-and-periodic-table-elements
Henry Moseley | Biography, Education, Discoveries, & Facts - Britannica, accessed March 28, 2025, https://www.britannica.com/biography/Henry-Moseley
Moseley's law - Wikipedia, accessed March 28, 2025, https://en.wikipedia.org/wiki/Moseley%27s_law
www.sas.upenn.edu, accessed March 28, 2025, https://www.sas.upenn.edu/~mabruder/moseleypage.html#:~:text=Moseley%20developed%20an%20apparatus%20to,series%20on%20the%20periodic%20table.
moseleypage, accessed March 28, 2025, https://www.sas.upenn.edu/~mabruder/moseleypage.html
Henry Moseley, X-ray spectroscopy and the periodic table | Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences - Journals, accessed March 28, 2025, https://royalsocietypublishing.org/doi/10.1098/rsta.2019.0302
Henry Moseley, X-ray spectroscopy and the periodic table - PubMed, accessed March 28, 2025, https://pubmed.ncbi.nlm.nih.gov/32811359/





Comments